Endoscopy - ScopeTrack
Just as with our other products, ScopeTrack enables full regulatory compliance, enhanced efficiency, cost saving and patient safety.
We continually add new functionality to provide departmental managers with access to quality management and efficiency reporting so that process improvements can be identified and actioned.
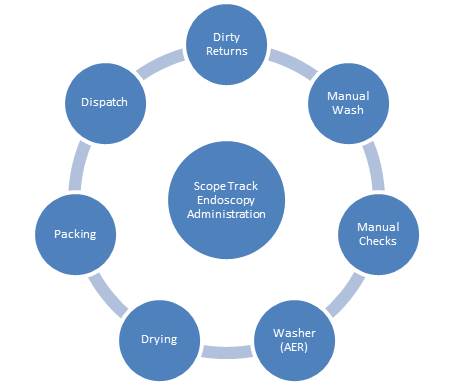
ScopeTrack covers the whole Endoscopy lifecycle from dirty return receipt through to despatch to treatment rooms. It also provides full patient association.
ScopeTrack integrates with a wide range of processing equipment, automates data capture and provides process validation data. This enhances patient safety, reprocessing speed and accuracy. More information on this is included in both Clinical Interfacing and Machine Integration options.
Recording consumables
We make it possible to integrate data relating to consumable usage in the process cycle. As a result, detailed management reports can be created to identify opportunities to reduce departmental expenditure. More information on this area can be found in our Departmental Management section.
To discuss your department’s needs in more detail, including integration into clinical management systems, please Contact Us
Please click on the following link for more details on how ScopeTrack can help you.
Solutions
- Sterile Services
- Endoscopy
- Theatres
- Consumable Items
- Machine Integration
- e-Learning Platform (ELP)
- MS1 Integration
- Resources & Datasheets
- OratoDat Insight
Brexit Statement
View FingerPrint Medical's Brexit StatementCORVID-19 Statement
View FingerPrint Medical's COVID-19 StatementContact Us
T: +44 (0) 1454 338 742 (UK)
T: +353 (0) 1685 3108 (Ire)
F: +44 (0) 1454 338 749
E: info@fingerprint.co.uk
Unit 7, Hatters Lane, Chipping Edge Estate, Chipping Sodbury, BS37 6AA
